2021 Patient Education Events & Global Health Repurposing Awards


Announcing our virtual Fall 2021 patient education events!
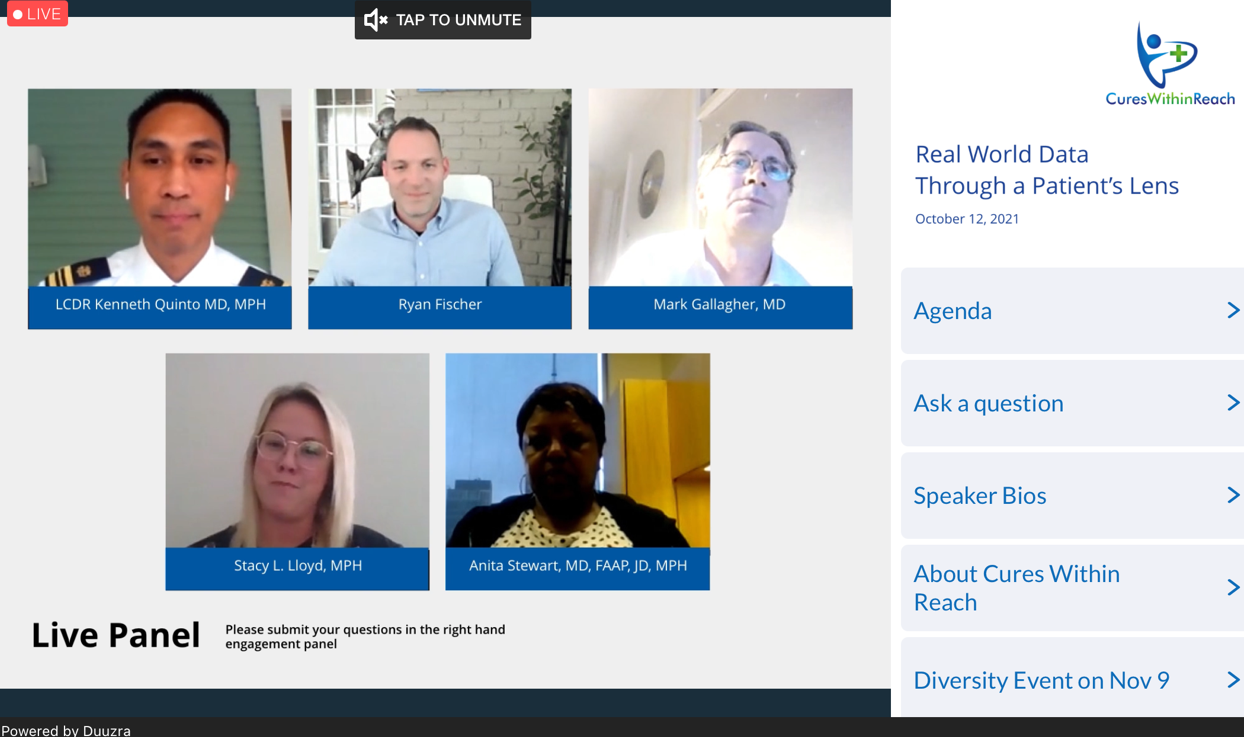
Real World Data Through a Patient’s Lens
October 12, 2021 from 1:00pm to 2:30pm ET
A conversation on Real World Data (RWD) and case reports from clinicians and researchers. This patient education event will focus on what RWD means, the importance of RWD in clinical decision making, RWD and off-label usage by doctors and patients, the role patients and caregivers can play in collecting RWD, and how patients and caregivers can utilize RWD to impact their own healthcare. Speakers will provide the perspectives of industry, payers, clinicians, nonprofits and the patient voice.
Moderator: Kenneth Quinto MD, MPH, Senior Medical Advisor for Real World Evidence Analytics, Center for Drug Evaluation and Research, US FDA
Speakers:
Ryan Fischer, Chief Advocacy Officer, Parent Project Muscular Dystrophy
Mark Gallagher, MD, Cardiologist, St George’s Hospital NHS Trust, UK
Stacy L. Lloyd, MPH, Director, Digital Health & Operations, American Medical Association, and Board Certified Patient Advocate
Anita Stewart, MD, FAAP, JD, MPH, Medical Director, BCBS of Illinois
Diversity of Clinical Trial Researchers and Patients: A Repurposing Opportunity?
November 9, 2021 from 1:00pm to 2:45pm ET
A dialogue on the dual opportunities of clinical trial diversity to impact health disparities and/or improve minority leadership in scientific research. Topics include the importance of diversity in finding treatments for unsolved diseases, health disparities and the role of repurposed therapies in addressing them; community organizations’ role in clinical research diversity; and the need to reduce the NIH funding gap for underrepresented racial/ethnic minority researchers. Speakers will provide the perspectives of industry, research, clinicians, community organizations and the patient voice.
During the event, there will be a showcase of our ongoing clinical trials within our Diversity, Equity & Inclusion Community, including projects impacting health disparities and led by racial/ethnic minority and underserved scientists.
Moderator: RADM Richardae Araojo, PharmD, MS, Associate Commissioner for Minority Health, Director, Office of Minority Health and Health Equity, US Food & Drug Administration
Speakers:
Regina Greer-Smith, MPH, LFACHE, President, Health Research Associates
LaShell Robinson, MS, Director, Diversity & Inclusion in Clinical Trials, Takeda Pharmaceuticals
Karriem S. Watson, DHS, MS, MPH, Chief Engagement Officer, All of Us Research Program, National Institutes of Health
Showcase Presenters:
Mainga Hamaluba, MBChB, MRCPCH, MD, KEMRI Wellcome Trust Research Programme
David Meltzer, MD, PhD, University of Chicago
Tycel Phillips, MD, Michigan Medicine

Thank you for joining us for these virtual Fall 2021 patient education events to help honor our 2021 Patient Impact Award Recipients.
Patients and caregivers FREE thanks to our supporters and partners. Nonprofit attendees only $15 for one event or $25 for BOTH events. Industry attendees only $30 for one event or $45 for BOTH events.
After both of our patient education sessions, Cures Within Reach presented ReFRAME at Scripps Research / Calibr, CURE ID at the FDA and NCATS/NIH and Eli Lilly and Company with their 2021 Patient Impact Awards, recognizing each organization’s outstanding achievements in creating impact for patients by repurposing existing science and medicine.
During these patient-focused events, Cures Within Reach celebrated and honored:
- ReFRAME at Calibr, Scripps Research’s drug discovery division, and the impact that its screening database is having on patients with neglected diseases as well as finding potential COVID-19 treatments. We celebrate Calibr’s commitment to the database’s ease of accessibility and the collaboration made possible through this technology, especially during the COVID-19 pandemic. Since ReFRAME’s launch, repurposed drugs such as clofazimine in cryptosporidiosis and auranofin in TB are impacting patients in clinical trials.
- CURE ID, a tool that allows clinicians and researchers to share repurposing case reports as a means to identify drugs for further study in clinical trials. We celebrate the development and launch of CURE ID for infectious diseases and soon for other diseases as well. We especially recognize the enhancements the FDA and NCATS/NIH made to CURE ID to help assist in the COVID-19 response. CURE ID is an example of the government’s critical role in the repurposing ecosystem and the FDA and NIH’s long-time commitment to enhancing drug development for difficult-to-treat diseases.
- Lilly’s ongoing commitment to improving the lives of patients with unmet medical needs. During the COVID-19 pandemic, this has been through its support of baricitinib, an existing rheumatoid arthritis medication, to treat certain hospitalized COVID-19 patients, among other initiatives. Baricitinib has been authorized for emergency use by the FDA in combination with remdesivir. In addition, Lilly has continued to test already approved therapies for new indications, including duloxetine for fibromyalgia and ramucirumab for various cancers after it was first approved for stomach cancer.
Congratulations to our 2021 Patient Impact Awardees:
Thanks to our Patient Education supporters, our Repurposing Community Industry Members, Philanthropic and Nonprofit Partners – all who help us provide free registration to patients and caregivers!











To learn about sponsorship opportunities, email GHRA@cureswithinreach.org
Janet Davison Rowley Patient Impact Research Award
The Janet Davison Rowley Patient Impact Research Award is given to one or more researchers, clinicians or computational biologists who have created positive patient impact through repurposing research. We honor the impact Dr. Rowley (1925-2013) had on patients through her clinical care and medical research, including her pioneering work that led to the creation of drugs that were later repurposed for additional indications.
Previous Awardees Include:
- ReFRAME at Calibr, Scripps Research
- SPARK at Stanford, Stanford University School of Medicine
- Dr. Denise Faustman, Massachusetts General Hospital and Harvard Medical School
- Dr. Scott Weir, The University of Kansas Cancer Center and the University of Kansas Medical Center (See a short video about his research at the top of this page!)
- Dr. Berish Rubin & Dr. Sylvia Anderson, Fordham University
- Dr. Marty St. Clair, Viiv Healthcare
- Dr. Vincent Rajkumar, Mayo Clinic
- Dr. James Surmeier and Dr. Tanya Simuni, Northwestern University
- Dr. David Teachey, Children’s Hospital of Philadelphia
The researcher, clinician, and/or computational biologist should fit the following profile:
- Has successfully repurposed a drug, device or nutraceutical for human clinical use, which is either used off-label or has been approved by the FDA or other regulatory agency; has made significant advancements in repurposing research; and/or is a recognized thought leader in repurposing research
- Has published repurposing research data in the scientific literature
- Patients who use or used a repurposed treatment discovered and/or advanced by the nominee enjoy an increased quality and/or length of life
- Will work with Cures Within Reach on public recognition of the award
Golan Christie Taglia Patient Impact Philanthropy Award
The Golan Christie Taglia Patient Impact Philanthropy Award is given to an individual, group or organization that has created positive patient impact by advocating for patients or contributing to the growth of repurposing research through financial, operational or professional philanthropic support. Named in honor of Golan Christie Taglia LLP in gratitude for their many years of financial and in-kind support for Cures Within Reach, helping us to grow and thrive.
Previous Awardees Include:
- CURE ID at the FDA and NCATS/NIH
- Drugs for Neglected Diseases initiative
- The Alzheimer's Drug Discovery Foundation
- The Leukemia & Lymphoma Society
- FRAXA Research Foundation
- Dr. Nicolas Sireau, with Professor Lakshminarayan Ranganath, AKU Society
- The Robert Wood Johnson Foundation
- George and Judy Goldman, Goldman Philanthropic Partnerships
- Golan Christie Taglia LLP
The individual, group, or organization selected should fit the following profile:
- Be a recognized leader in an advocacy, research, healthcare, philanthropic or non-governmental (NGO) organization endeavor, or be a supporter of such an endeavor
- Has made significant philanthropic contributions and/or created fundraising that have resulted in the advancement of repurposing
- Has brought together various stakeholders to create an increased quality and/or length of life for patients
- Will work with Cures Within Reach on public recognition of the award
Patient Impact Industry Award
The Patient Impact Industry Award is given to an industry individual, group or organization that has created positive patient impact by contributing to the growth and profile of repurposing research within the healthcare industry, through financial, operational and/or collaborative support. Nominees may represent any size of industry organization, from start up to large pharmaceutical companies. In addition, nominees may also represent any sector that impacts repurposing research, including biotech, pharma and indutry service / support providers.
Previous Awardees Include:
- Eli Lilly and Company
- Camargo Pharmaceutical Services
- Jazz Pharmaceuticals
- Novartis
- Timothy P. Walbert, Horizon Therapeutics plc
- Dr. Jerome B. Zeldis, Sorrento Therapeutics, formerly of Celgene Global Health and Celgene Corporation
- Dr. Richard DiMarchi, University of Indiana, formerly of Eli Lilly
- Dr. Norbert Riedel, Aptinyx Inc., formerly of Baxter International
- Michael S. Rosen, Rosen Bioscience Strategies, formerly of Illinois Science + Technology Park and Forest City Realty Trust
The individual, group, or organization selected should fit the following profile:
- Is or was in a significant leadership position in the healthcare industry and has demonstrated a commitment to collaboration, service and/or support for repurposing research
- Focused the attention of the healthcare industry, and/or their own group or organization and similar groups or organizations, on improving the lives of patients through repurposing
- Patients who use or used a repurposed treatment supported by this nominee enjoy an increased quality and/or length of life
- Will work with Cures Within Reach on public recognition of the award
Questions? Contact Clare Thibodeaux, PhD, Vice President of Scientific Affairs: clare@cureswithinreach.org or 847-745-1250


